

Journal of Multidisciplinary Dental Research
Volume: 4, Issue: 2, Pages: 5-12
Original Article
Raghunath Puttaiah1, Jason A. Griggs2, Chadwick Sargent3, Sanjay Parekh4
1Diagnostic Sciences, Texas A&M University College of Dentistry, Dallas, Texas, USA
2Department of Biomaterials Science, Mississippi State University Medical Center
3Endodontist, Lubbock, Texas, USA (ex-student of Texas A&M College of Dentistry)
4Orthodontist, Ashland, Kentucky, USA (ex-student of Texas A&M College of Dentistry)
Corresponding
Raghunath Puttaiah
Professor, Diagnostic Sciences, Texas A&M College of Dentistry
3302 Gaston Avenue, Dallas, TX 75246
Tel: 214-828-8245 Fax: 214-828-8306
Received Date:12 September 2018, Accepted Date:22 September 2018, Published Date:23 September 2018
OBJECTIVES: A variety of agents have been evaluated for efficacy as waterline sanitizers. One concern is the possible reduction in the bond strength of dental composites to dentin when a constantly present antimicrobial/cleaning agent is used in dental unit waterlines as an irrigant. The purpose of this study was to determine if the use of various sanitizers versus municipal tap water resulted in a difference in the bond strength of composite to dentin.
METHODS: Non-carious third molars were invested in die stone and sectioned. 14 teeth were randomly allocated to be treated with each of the following irrigants: BioClear, Bio 2000, Oris CHX, CloSYS II, DentaPure, DioxiClear, and municipal water from the City of Dallas, Texas. The teeth were acid etched, rinsed with the irrigant dependent on the treatment group, conditioned with a bonding agent, and bonded to a composite button. The specimens were thermally cycled, and each composite button was mechanically loaded until debonding occurred. Debonded surfaces were classified according to failure mode. The irrigants were tested for difference in mean failure loads using one-way ANOVA at α=0.05.
Results:There was no significant difference in mean failure load based on irrigant (p = 0.07). The debonded surfaces followed a trend of fewer adhesive failures with increasing mean failure load. Significance. These results indicate that the presence of the agents examined in dental waterlines will not affect the ability of dentin bonding while using Herculite (KERR-SDS).
Keywords:dentin bonding, antimicrobial agents, irrigants, waterline cleaners, fractography, survival analysis.
Dental unit water systems that draw directly from municipal water and those that have a selfcontained water reservoir system are normally contaminated with high levels of microorganisms that may be pathogenic or non-pathogenic. The source of the microbes being introduced into the line may be from patients' oral cavities or from source water of poor quality. Over a short period, the water system develops biofilms that coat the inner surfaces of the system and amplify the contamination in the dental treatment water, commonly exceeding one million colony-forming units per milliliter (CFU/mL).1 While this problem has been known for the past few decades, the American Dental Association and the United States Centers for Disease Control and Prevention has set up a goal to control this contamination. In simple terms the goal indicates that the dental treatment water to be used in a patient's oral cavity during non-surgical care should contain equal to or less than 500 CFU/mL.2
The many dental water treatments and biofilm control methods available in the market can be classified as either physical or chemical methods. The physical methods are point-of-use membrane filters (with or without endotoxin control) on individual water lines. The chemical methods include the use of continuously/constantly present antimicrobial agents in the lines to control planktonic microbial contamination of the water/irrigant, and the periodic cleaning of lines with a stronger germicide/cleaner to control or remove attached biofilms within the waterlines, immediately followed by purging with microbe free water. Materials that are used as periodic cleaning agents may or may not leave residue, based on the indicated amount of rinse water to displace the agent. One concern is possible reduction of the bond strength of composite restorations to dentin when a constantly present antimicrobial/cleaning agent is used in the water system as an irrigant.
Several previous studies have investigated related effects. Williams and Von Fraunhofer studied the effect of various irrigating solutions on the bond strength of a fissure sealant to enamel. They reported no difference between specimens rinsed with tap water, hard water, deionized water, and fluoridated water3. However, rinsing with 1% potassium chloride or sodium chloride solution increased bond strength. Discharging the prepared enamel surface with a grounded electrical wire produced a similar increase in bond strength. The authors concluded that the increased electrical conductivity of the salt-containing irrigants allowed elimination of static charge and improved surface wetting.
Chlorhexidine-based disinfectants have received strong interest with regard to their effect on dentin bonding and micro leakage4-7. The literature contains conflicting findings that a 2% chlorhexidine rinse both does and does not adversely affect the bond strength of composites to dentin. Chlorhexidine has been reported to increase the surface energy of human enamel8. A similar action on the demineralized dentin layer would be expected to improve bonding.
Pegoraro et al. measured the bond strength of NiCr full crowns to teeth cleaned with a detergent (sodium sulfate lauryldiethyleneglycol ether), detergent plus 32% polyacrylic acid, and detergent plus 50% citric acid9. The crowns were cemented with either a zinc phosphate or a zinc polycarboxylate luting agent. Pegoraro et al. found no difference in bond strength based on cleansing agent or luting agent.
Roberts et al. investigated the effect on shear bond strength of rinsing with distilled water, a 3 ppm sodium hypochlorite solution, a residual chlorhexidine/ethanol solution (Bio 2000, Micrylium Labs), a 0.224% citric acid solution (BioClear, Waggoner Product Development), and a 1:10 dilution of Listerine (Warner-Lambert)10. They reported that all the sanitizers resulted in lower bond strength values than those achieved using distilled water. This difference was statistically significant for only chlorhexidine /ethanol and Listerine rinses. The distilled water used as a control by Roberts et al. may provide different bond strength than municipal tap water, latter, commonly used in dental practice. Also, a variety of other agents have recently been evaluated for efficacy as waterline sanitizers11-14.
The purpose of this study was to determine the effect of various dental unit waterline cleaning and antimicrobial agents constantly present in the waterlines as irrigants on the shear bond strength of a dentin bonding restorative agent. It was hypothesized that no difference in bond strength would result from rinsing with cleaning and antimicrobial agents versus municipal water.
Materials and methods
Materials. The sanitizers included in this evaluation were 0.21% citric acid (BioClear, Waggoner Product Development, Dallas, Texas, USA), 0.12% chlorhexidine gluconate/12% ethanol (Bio 2000, Micrylium Labs, Toronto, Canada), 1:20 dilution of a 0.12% chlorhexidine gluconate/12% ethanol (Oris CHX, Dentsply International, York, Pennsylvania, USA), 1:10 dilution of a stabilized chlorine dioxide (CloSYS II, Rowpar Pharmaceuticals, Scottsdale, Arizona, USA), 4 ppm of iodine in municipal water (DentaPure, MRLB International, Fergus Falls, Minnesota, USA), 3 ppm of Chlorine Dioxide in municipal water (DioxiClear, Frontier Pharmaceuticals, Melville, New York, USA), and municipal water (City of Dallas, Texas, USA) that had a hardness of about 150-200 ppm of particulate matter as a control. The United States Food & Drug Administration (US-FDA) and/ the United States Environmental Protection Agency (US-EPA) have not approved/registered some of the materials tested in this study as devices for patient use within their jurisdiction. Some of these materials were marketed as cleaners with no antimicrobial claims and therefore at the time of the study were not registered with the US-EPA as a pesticide/germicide or had obtained clearance for market by the US-FDA.
Tooth preparation. Each treatment group and the control group comprised of 14 human third molars (mandibular and maxillary at random). These teeth were intact, non-carious, and free of developmental defects prior to extraction. The teeth were debrided of soft tissue and preserved in 0.05% thymol solution. Figure 1 is a step-by-step template that provides the sample preparation procedure per tooth. The teeth were then placed in a metal sleeve (transverse cut pipe), invested in dental die stone (Die-Keen), and set in a humidor for 40 minutes at 100% humidity. Die stone was chosen as a mounting material to avoid contamination of the dentin surface with resin during sectioning and polishing. The occlusal enamel was removed after mounting to expose the dentin using a water-cooled, low-speed diamond saw (Isomet, Buehler, Lake Bluff, Illinois, USA). The dentin surface was flattened using 600 grit silicon carbide abrasive paper (20 30-cm long strokes). The teeth were then be examined at 8X magnification using an optical stereomicroscope (Stereomicroscope SR, Carl Zeiss, Thornwood, New York, USA) to ensure that all enamel was removed. The teeth were stored at room temperature (23 ± 2C) in distilled water.
Bonding. Fourteen teeth were allocated randomly to each treatment group and one control group (98 teeth total) for bonding. Each waterline irrigant was loaded into a self-contained reservoir of an ADEC dental unit (ADEC, Newberg, OR) and this irrigant was used during the bonding procedure for the 14 teeth belonging to one group. After being dried for 10 seconds with oil free, compressed air, a 35% phosphoric acid etchant (UltraEtch, Ultradent Products, South Jordan, Utah, USA) was applied for 15 seconds to the dentin surface of each tooth. The etchant was removed by a 5-second rinse with the given irrigant. Excess of the irrigant was dabbed with a 4 X 4 inch cotton gauze square twice quickly leaving the surface still moist and glossy. The bonding agent (Optibond Solo, KERR-SDS, Orange, California, USA) was applied with a disposable syringe tip to the dentin surface for 15 seconds with a continuous scrubbing motion. A 2second jet of compressed air was used to thin the material. The dentin surface was then visually inspected to ensure that it remained glossy. The specimen surface was activated with a light unit (Optilux 500, Demetron Research Corporation, Danbury, Connecticut, USA) for 20 seconds. The adequacy of light intensity in the 540-570 nm wavelength range was assessed using the built-in radiometer. A stainless steel washer (3.85-3.90 mm ID and 1 mm thickness) was placed on the dentin surface. A dental composite (Herculite, KERR-SDS, Orange, California, USA) was inserted into the inner diameter of the washer and light activated by exposure for 40 seconds to the light unit after each increment.
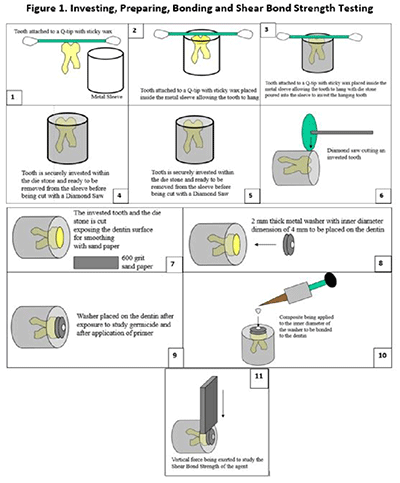
Manual cycling and aging of specimen. The bonded specimens, including tooth, cured composite, washer, and die stone, were thermally o ocycled in a custom apparatus from 5C to 55C in water for 500 cycles.
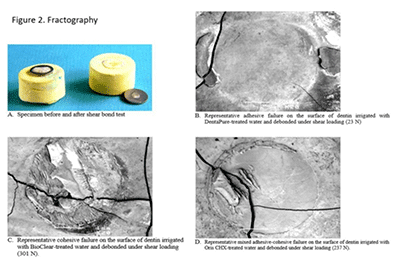
Shear bond strength determination. The specimens were mounted in a custom jig and loaded to failure in shear using a mechanical test machine (Model 1125, Instron Corporation, Canton, Massachusetts, USA)15. A steel blade was placed on the rim of the washer parallel to the tooth surface allowing the washer to distribute the loading force around the composite button. The specimen was loaded at a constant crosshead speed of 0.254 mm/min until a maximum in the load-deflection curve was recorded. Figure 2A shows specimens before and after shear bond test. In some cases, the die stone mount fractured prior to dentin-composite debonding, resulting in a load-deflection curve with two maximum load values. In each of those cases, the first maximum value was recorded, and it was treated as a right censored value in the statistical analysis.
Fractographic analysis. The debonded surfaces examined at 50X magnification using an optical stereomicroscope. The fractured areas were categorized by mode of failure. Each crack path was either entirely along the composite-tooth interface (adhesive failure), within the bonded materials (cohesive failure), or a mixture between these two failure modes (mixed failure). Also, any outstanding fracture surface topography, such as dentin cracking and pulp chamber breaching, was noted. In the case of a pulp chamber breach, the specimen in question was not included in the data set.Statistical methods. The mean failure load and standard deviation for each group was calculated using the method of Kaplan and Meier. for right censored data16. The mean failure loads for the six treatment groups and the control group were tested for a significant difference using one-way ANOVA (a = 0.05)
RESULTS
Seven out of 98 specimens (7%) debonded during the thermal cycling treatment and were not mechanically loaded. The die stone mounting cracked prior to debonding in 28 (29%) of the specimens. Treating these observations as right censored data resulted in the following mean failure loads and standard deviations: 241 ± 75 N (BioClear), 234 ± 70 N (CloSYS II), 219 ± 68 N (Oris CHX), 173 ± 89 N (Dallas municipal water),170 ± 61 N (Bio 2000), 165 ± 86 N (DioxiClear), and 161 ± 127 N (DentaPure). These data are summarized in Table 1. One-way ANOVA showed no significant difference in mean failure load between irrigants (p = 0.07).
Table 1
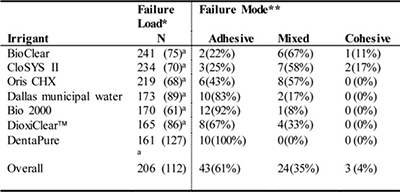
*mean (standard deviation), **number observed (proportion)
*not significantly different according to one-way ANOVA ( = 0.05)
Seventy specimens were debonded without a failure in the mounting material. Of these, 43 (61%) were classified as adhesive failures (Figure 2B), 3 (4%) were classified as cohesive failures (Figure 2C), and 24 (35%) were classified as mixed failures (Figure 2D). Table 1 shows the distribution of these categories for each irrigant. The fractured surfaces showed a trend between irrigant groups of increasing proportion of adhesive failures with decreasing mean failure load. Figure 2 shows a representative debonded surface from a DentaPure-treated specimen with an entirely adhesive failure. Figure 2B provides an example of an entirely cohesive failure (Bioclear), and Figure 2D shows a surface with a mixture of adhesive and cohesive failure (Oris CHX). As Figures 2B, 2C and 2D illustrate, dentin cracking and composite gouging frequently occurred, but dentin gouging was relatively rare.
Discussion
The range of shear bond strength values that were observed in the present study are within the same range or slightly lower than those reported for the same materials by previous investigators17,18. The diameter of the bonded area on each specimen was approximately 4 mm, and the bonded area was 2approximately 12.6 mm. Although the applied force is not distributed evenly across this area19 . an average shear stress can be calculated by dividing the applied load by the bonded area. The result is mean failure stresses ranging from 12.8 to 19.2 MPa in the present study. Hübsch et al. used finite element modeling to estimate the stress present at the composite-tooth interface of bulk fillings under static loading20. They predicted maximum shear stress levels of 8 to 9 MPa. All mean bond strengths observed in the present study are sufficient to withstand these predicted levels of stress.There was a clear difference in fracture patterns between debonded surfaces that failed in adhesive, cohesive, and mixed failure modes. Furthermore, the frequency of adhesive failure was distinctly different between irrigant groups. Two studies also reported deviation of the debonding crack from a planar path along the interface may be caused by factors other than high bond strength and support this study's data, type and appearance of debonded surfaces was so consistent within each treatment group.21, 22 There was no significant difference in mean failure loads because of the high variance of the failure load data. Therefore, failure mode seems to be a more sensitive response variable than failure load for determining the quality of dentin bonding. However, even the irrigant group with 100% adhesive failures (DentaPure) exhibited a clinically acceptable mean failure load, so the additional sensitivity provided by failure mode analysis does not seem clinically useful2.
Graph-1
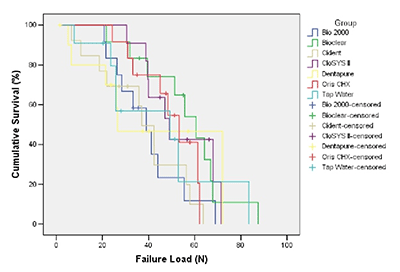
Graph 1 demonstrates that the results of the Kaplan-Meier survival analysis showing the percentage of specimens in each group that remained surviving at each level of interfacial stress. The + symbols indicate specimens that were censored at the stress level where the specimen holder (die stone) fractured.
Table 2 describes the ingredients, properties and relative efficacy of microbial control including biofilm control. Some of the properties of the cleaners and ingredients therein may have deleterious effects on bonding. Surfactant and wetting agents that are present in the cleaners may alter bonding as they may leave a residue. Other electrochemical properties such as pH and conductivity of the solution may also have deleterious effects. If the pH is low, there may be a continuous etching action that may increase the bonded surface area. This study did not evaluate the prepared surfaces using a scanning electron microscope, and therefore, we are not aware of the effects on etching and smear layer removal.
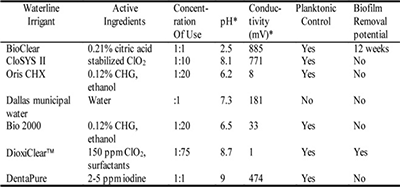
The cleaning and antimicrobial agents used in this study have been investigated for efficacy in controlling biofilm formation11-14. All items used as irrigants other than Dallas Municipal water have shown positive effects of controlling planktonic contamintion to less than 500 CFU/mL. Only BioClear and DioxiClear have been shown to remove biofilm, with DioxiClear removing biofilms in less than six weeks while BioClear removes them about 12 weeks. Considering the lack of influence of waterline irrigant on dentin bond strength, it is recommended to choose a cleaner based on efficacy and biocompatibility.
Die stone was chosen as the mounting material in the present study to prevent contamination of the dentin surface with resin during the cutting and polishing operations. Unfortunately, the die stone could not withstand the thermal cycling treatment used for accelerated simulation of aging in the oral environment. This resulted in increased variability in specimen investment diameter and failure of many specimen investments prior to debonding. The use of die stone for specimen fabrication is not recommended in future dentin bonding studies.
Conclusion
The results of this study indicate that the presence of the cleaning and antimicrobial agents that were examined in dental waterlines will not affect the ability of Herculite to bond to dentin when compared with waterlines containing only Dallas municipal water.
Acknowledgments
This study was supported in part by the Department of Diagnostic Sciences and the Department of Biomaterials Science, Texas A&M College of Dentistry. The materials were supplied by DentaPure, Micrylium Laboratories, Frontier Pharmaceuticals, Waggoner Development Corp.,Dentsply Preventive Care Division, Rowpar Pharmaceuticals, and KERR-SDS. The teeth were supplied by Dr. Mark Waggoner. We sincerely thank Dr. David Charlton and Dr. Howard Roberts, United States Airforce Dental Investigation Service, Great Lakes, IL for their help thermal cycling of the specimens in this study. The views expressed in this article are those of the authors and do not reflect the official policy of the United States Air Force, United States Navy/Naval Reserves or other departments of the United States Government.
Subscribe now for latest articles, news.