Journal of Multidisciplinary Dental Research
Volume: 4, Issue: 1, Pages: 48-55
Review Article
Rashmi S Pattanshetty1,B. S. Jagadish Pai2, Jayaprakash G.S3, Amit Walvekar4
1Postgraduate student, Dept. of Periodontics, Coorg Institute of Dental Sciences, Virajpet.
2Professor and HOD, Dept. of Periodontics, Coorg Institute of Dental Sciences, Virajpet.
3Reader, Dept. of Periodontics, Coorg Institute of Dental Sciences, Virajpet.
4Professor, Dept. of Periodontics, Coorg Institute of Dental Sciences, Virajpet.
Received Date:12 October 2018, Accepted Date:30 October 2018, Published Date:10 November 2018
Periodontal diseases with their polymicrobial etiology, are a major cause of tooth mortality in the adult population. Current treatment modalities have resulted only in arresting the disease progression but have not cured the disease completely, nor do they prevent the recurrence.
Hence there is a need for more sophisticated therapeutic modalities which may include vaccines targeting putative periodontal pathogens. No periodontal vaccine trials have been successful in satisfying all requirements of an ideal periodontal vaccine. Periodontal vaccines could emerge as an adjunct to mechanical therapy in future.
Key words: Periodontitis, immunity, vaccine.
Vaccine is name applied generally to substance of nature of dead or attenuated living infectious material introduced into the body with object of increasing its power to resist or get rid of a disease. Vaccination is the best known and the most important application of immunological principles to human health.
Vaccines are generally prophylactic i.e., they ameliorate the effects of future infection.1
HISTORY
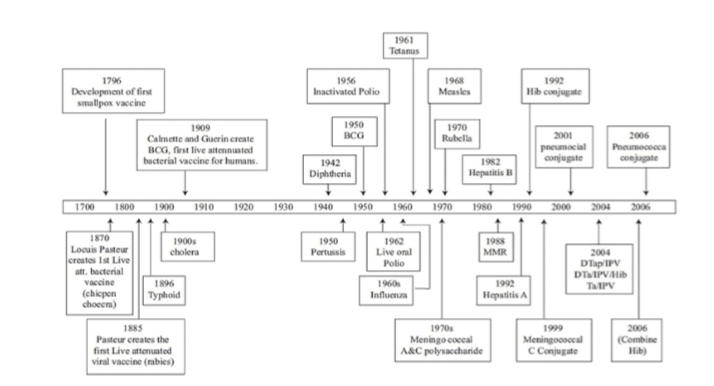
The first vaccine was named after Vaccinia, the cowpox virus. In the late 18th century, Edward Jenner developed and established the principle of vaccination using the cross protection conferred by cowpox virus. In 1885 the French microbiologist Louis Pasteur developed first vaccine against rabies. Even Pasteur did not have a proper understanding of immunological memory or the functions of the lymphocyte, which had to wait another half century. Finally with Burnet's clonal selections theory (1957) and the discovery of T and B lymphocytes (1965), the key mechanism became clear.
HOW IMMUNITY DEVELOPS?
Specific immune response2
Chronic inflammation, if protracted, can result in an adaptation called the specific immune response. The specific immune response requires lymphocytes which use 2 types of receptors to generate specific immune responses, the b-cell antigen receptor and the t- cell antigen receptor.
Four phases are involved in the generation of specific immunity:
Clonal selection: Selection of lymphocytes that bear receptors recognizing the specific antigen
Clonal expansion: Proliferation of those lymphocytes
Clonal contraction: Death of effector Lymphocytes
Memory: Maintenance of an expanded clone of cells that bear the specific receptors recognizingthe antigen.
As long as a sufficient number of lymphocytes are maintained to provide protection against a specific pathogen, the individual is said to be immune. 'Vaccination is the development of immunity or resistance to infection, after a secondary response (booster) that is adequate to consider the individual immune to a subsequent infection.’
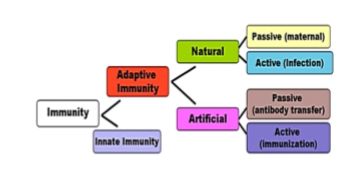
TYPES OF VACCINATION
Active immunization - in which an individual immune system is stimulated by administrating killed or live attenuated products derived from micro-or ganisms.3
Passive immunization in which the antibodies formed in an individual, is transferred to another. DNA vaccination in which DNA plasmids encoding genes required for antigen production are transferred to an individual.
KEY FEATURES OF A SUCCESSFUL VACCINE
Safety
Protective
Should provide sustained protection
Should produce neutralizing antibodies
Should stimulate protective t-cells
Practical considerations like
Cost effect
Biological stability
Access4
NEED FOR DEVELOPMENT OF PERIODONTAL VACCINE
The primary aim in introducing periodontal vaccine is to eradicate the periodontal disease burden. The vaccine should positively enhance the maintenance of oral health and optimise the retention of the natural status thus decreasing the need for prosthesis in the oral cavity.
Moreover, recent findings link periodontitis and systemic health concerns like atherosclerosis, diabetes mellitus, pre-term low weight birth, rheumatoid arthritis suggest that prevention or treatment of periodontal diseases is a baseline step for the effective management of atherosclerosis, uncontrolled diabetes, and low weight pre-term birth or preeclampsia.
Also to reduce financial burden which periodontal disease puts on patient.5
HISTORY OF PERIODONTAL VACCINES
In the early 20th century, 3 periodontal vaccines were employed.
These were:
Pure cultures of streptococcus and other organisms
Autogenous vaccines
Stock vaccines
Eg.Vancott's vaccine
Inava endocarp vaccine
The search for the etiologic agents of periodontal disease and the vaccines ended inconclusively; probably the most important reason for the failure was the inability to conduct adequately controlled clinical trials and experiments.6
TYPES OF PERIODONTAL IMMUNIZATION
Active immunization
Whole bacterial cells
Sub unit Vaccines
Synthetic peptides as antigens
Passive immunization
Murine monoclonal antibody
Plantibodies
Genetic immunization
Plasmid vaccines
Live, viral vector vaccines
Active immunization
Whole cells
The entire cell with its components is inoculated into a host to bring about active immunization (a)Klausen in 19917 has shown that levels of serum antibodies to both whole cells and partially purified fimbriae from P. gingivalis were elevated in rats immunized with P. gingivalis cells.
(b)Kesavalu in 19928 observed protection against invasion, but no colonization against P. gingivalis in a mouse chamber model by immunization with either killed heterologous invasive or noninvasive P. gingivalis strains. The immune response to whole cells or selected envelope component did not completely abrogate lesions, but eliminated mortality.
P . gingivalis, Tanerella forsythia, Treponema denticola have been consistently and strongly associated with progression of disease, suggesting that these four bacterial species may be the major pathogens of periodontitis in human.9
P. gingivalis has emerged as the leading candidate pathogen in the development of chronic periodontitis. It is a gram-negative, nonspore/forming, nonmotile, assacharolytic, obligate anaerobic coccobacillus.10
The virulence factors of P. gingivalis which have been used as subunits for the development of active immunization are:11
Outer membrane protein
It was seen that transcutaneous injection of 40 kDa of outer membrane protein (OMP) inhibits coaggregation of P. gingivalis with Streptococcus gondonii.
This also can be used for vaccine development for passive immunization. Polyclonal anti-40 kDa.OMP antibody exhibited potentially protective, complement-mediated bactericidal effect.12
Gingipains13,14
Coined by Travis and Colleague
These are cysteine proteinases which cleave synthetic and natural substrates after arginine or lysine residues and are referred to as arginine gingipain (Rgp) and lysine gingipain (Kgp), respectively.13-16
HRgpA (90kDa) and RgpB(50kDa) products of 2 distinct but related genes rgpA and rgpB respectively are specific for Arg-Xaa peptide bonds.
HRgpA and Kgp are non-convalent complexes containing separate catalytic and adhesion/ hemaggulutin domains while RgpB has only catalytic domain.17
HRgpA and RgpB induce vascular permeability enhancement through activation of kinin pathway and activate the blood coagulation system which is associated with GCF production and progression of inflammation leading to alveolar bone loss in periodontitis site.Kgp is most potent fibrinogen degrading enzyme of 3 gingipains in human plasma and involved in bleeding tendency at diseased gingiva.
They are expressed on the outer membrane of P. gingivalis. Rgp and Kgp are key determinants in the growth and virulence of P. Gingivalis.
Gingipains vaccines are mainly DNA vaccines.DNA vaccines induce both humoral and cellular immunity.
Fimbriae
Fimbriae from P.gingivalis play an important role in adhesion to oral tissue and are highly immunogenic. Chan in 1995 demonstrated that immunization with purified outer membrane protein reduces the activities of collagenase, gelatinase and cysteine proteases in gingival tissue.18
These are cell surface structure components and serve as a critical antigen. These are the most advanced immunogens.
Functions of fimbriae are the following:
Currently, five P. gingivalisfimbrial types (I-V) have been described based on their antigenicity. However, a vaccine based on one fimbrial type may be strain specific and hence ineffective against other P. gingivalis strains of different fimbrial types.
GroEL heat shock protein
Heat shock proteins have an important role in inflammatory mechanism, autoimmune disease and atherosclerosis. Homologues of specific stress protein families have been demonstrated to be present in oral bacteria including Fusobacteriumnucleatum, Prevotellaintermedia, Prevotellamelaninogenica, A. comitans and P. gingivalis.Rats immunized with P. gingivalis HSP60 showed decrease in bone loss induced by infection with multiple periodontopathic bacteria.Significant association between HSP90 concentration and microbial colonization has been observed.20
Hemagglutinins
Non-fimbrial adhesion hemagglutinin B (HagB) is a potential vaccine candidate. Hemagglutinin mediates bacterial attachment and penetration into the host cells, as well as agglutinates and lyses erythrocytes to intake heme, an absolute requirement for growth.Mice intragastrically inoculated within a virulent strain of Salmonella typhimurium expressing HagB gene mounted both systemic and mucosal antibody response and this response could be boosted indicating that a memory T-cell or B-cell response was induced.Furthermore, rats immunized subcutaneously with recombinant HagB were protected against periodontal bone loss induced by P. gingivalis strain ATCC 33277.Human antibody against hemagglutinin should be ideal for practical use in immunotherapy.21
Synthetic peptides
These require synthesis of linear and branched polymers of 3-10 amino acids based on the known sequences of microbial antigens. Such peptides are weakly immunogenic by themselves and need to be coupled to large proteins to induce antibody response.
Two ways of developing synthetic peptide vaccines are as follows:
By deduction of the protein sequence of microbial antigens from RNA sequence data.
By testing overlapping peptides and by mutational analysis.
Advantages of synthetic peptide are:
SafeCheap
Easy to store and handle
Ideally suited for specific targeting, which is not possible with classical vaccines.
Genco in 1992 found that synthetic peptides based on the protein structure of fimbrillin inhibit the adhesion of Pg to saliva-coated hydroxyapetite crystals in vitro.22
Summary of findings from animal study models for active immunization vaccine trials in periodontitis
Passive immunization
Passive immunization is short lived, because the host does not respond to the immunization and protection lasts only as long as the injected antibody persists.
Here, the antigens are injected into a vector which produces antibodies. These antibodies, when inoculated into a host bring about passive immunization. Passive immunization can be brought about in two ways:
Murine monoclonal antibodies23
In this, the antibodies are obtained by inoculating the antigens into mice. These antigens are then injected into the host which brings about passive immunization.
Booth et al (1996) developed a murine monoclonal antibody to P.gingivalis which prevented recolonization of deep pockets by this pathogen in periodontitis patients.
Plantibodies
A very recent approach for vaccination strategies is molecular biological techniques to express bacterial or viral antigens in plants, which could be used as orally administered vaccines.
Ma et al (2000), characterized a secretory IgG antibody against Streptococcus mutans produced in transgenic plants.24
Advantages
More stable
Exhibited a higher function and
Protection against colonization by S mutans.
Genetic immunization
By the early 1990's, scientists had begun to study new approaches to the production of vaccines that differ in structure from traditional ones. The strategy involves genetic engineering, or recombinant DNA technology.
There are 2 types:
Plasmid vaccines25
DNA does not have the ability to grow, whereas plasmids can grow. With this ability of the plasmids, they are fused with the DNA of a particular pathogen of interest and inoculated in an animal for the production of antibodies. This is then transferred to the host for immunization.
Disadvantages of plasmid vaccines are that, in some cases it may lead to oncogenesis.
Live, viral vector vaccines
A variety of infectious but non-disease causing DNA or RNA viruses or bacteria have been engineered to express the proteins of a disease producing organism. The vector enters the body cells where the proteins are generated and then induce humoral or cellular immune responses.
Methods of DNA vaccine administration
intranasal
intramuscular
gene gun
Advantages of DNA vaccines
Can be manufactured more easily
Stable by nature
Simple26
Following are the summary of findings from human studies on serum titre responses to therapy or passive immunization in subjects with periodontitis.
Limitations of periodontal vaccine
The development of vaccines is a complex process that requires substantial resources over a long period of time.
Human periodontal disease is multifactorial caused by manifold pathogens. The multiplicity of pathogenic organisms indicates that vaccine design against periodontitis is very complex.
Bacterial whole cells or crude extracts preparation for vaccination is not desirable because the antigenic determinants of bacteria potentially possess a high risk of cross reactivity with human counterparts. Finally, animal models for vaccine trials may pose inconsistencies with human models in major histocompatibility complex restriction of antigens presented by antigen presenting, thus obscuring the immunodominant epitopes.
As an innovative strategy, vaccines using crossreactive immunodominant epitopes as antigenic molecules in an attempt to stimulate antigen-specific regulatory T-cells (Tregs, CD4+,CD25+, FoxP3+), secreting IL-10 and 10 and Transforming growth factor-β, may provide new clues for periodontal disease prevention, through the induction of either immune tolerance or an effector function.27
CONCLUSION
Vaccination may be an important adjunctive therapy to mechanical debridement in near future. It is not a myth but a reality which will come true in the near future if research is carried out in right way in right direction.
Pattanshetty et al., Periodontal vaccine a review.2018:4(1);48-55
Subscribe now for latest articles, news.